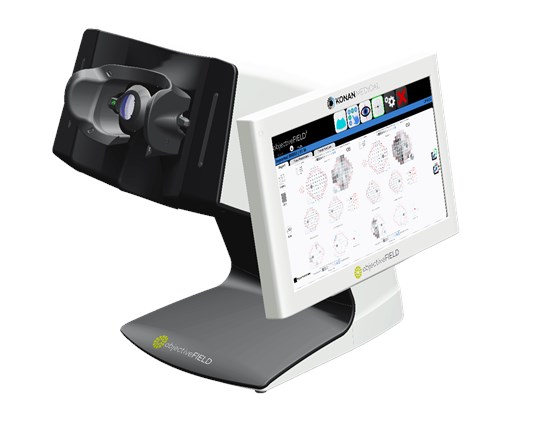
objectiveFIELD is the first and only truly objective perimeter FDA 510(k) cleared to assess visual field abnormalities
Irvine, California--(Newsfile Corp. - October 10, 2025) - Konan Medical USA, Inc. today announced its groundbreaking objectiveFIELD Analyzer (OFA®) has received CE Marking under the European Medical Device Regulation (MDR). This significant milestone enables the Company to offer advanced perimetry to healthcare providers across the European Union.
Receiving CE Marking signifies that the device meets the stringent safety and performance requirements set forth by the European Union, ensuring that it is reliable, effective, and safe for clinical use.
objectiveFIELD is also available for sale in the USA, Canada, Japan and select international markets.
OFA is a completely objective, bilateral visual field analyzer that measures pupillary responses to novel spatially resolved stimuli, captured by video cameras under infra-red conditions. Pupil responses are measured to assess visual field abnormalities instead of the subjective, push-button human-response to the small white-on-white flash of light used in subjective standard automated perimetry (SAP).
Retinal sensitivity is derived from the amplitude of the pupil responses, and OFA also measures delay/latency (time-to-peak constriction), which is new information that no other SAP device can provide. Additionally, SAP is a Half-Axis device in that it only measures reduced sensitivity defects whereas OFA is a Dual-Axis device that also reveals high-sensitivity defects with no extra effort or time.
Reports are presented in a familiar format for quick and easy interpretation.
"We are thrilled to have achieved CE Marking for the OFA. It is a testament to our team's dedication to innovation and developing novel medical technology," said Simon Gordon, co-Chief Executive Officer of Konan Medical USA, Inc.
The approval was granted after a rigorous assessment process, which confirmed that the OFA conforms to the requirements of the MDR Regulation EU 2017/745. This process involved a thorough review of the device's clinical data, technical documentation, and manufacturing processes to ensure that it meets high standards of safety and effectiveness. Konan Medical USA's quality system is certified to ISO 13485:2016 MDSAP.
"This approval is pivotal for expanding access to new, objective visual -function testing, which we believe will augment current standard of care and enable testing patients that are too young for traditional subjective methods. With test-protocols as fast as 90 seconds for both eyes, and no button to push, we expect clinics to will see improvements in efficiency and patient satisfaction. There is a great deal of initial interest from specialists that are focused on diabetic retinal disease (DRD) and pediatrics," said Dale Sadlik, co-CEO.
The OFA is also listed with FDA and is expected to be made available to European hospitals and clinics starting in October 2025.
For more information about objectiveFIELD including 39 peer reviewed clinical and scientific publications please visit https://konanmedical.com/objectivefield/.
objectiveFIELD and OFA are registered trademarks in U.S. Patent and Trademark Office.
To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/11937/269836_ce7fb015a4950ac8_001full.jpg
About Konan Medical USA, Inc.
Headquartered in Irvine, California, Konan Medical USA Inc. develops specialized diagnostic products for eye care providers. The company also provides diagnostic equipment, rental programs and support services to many of the leading global ophthalmic manufactures in support of sponsored clinical trials.
For more information about the Company please visit https://konanmedical.com.
Contact:
Ian McMillan
VP of Sales and Marketing
imcmillan@KonanMedical.com
###

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/269836