Articles published by Abiomed, Inc.
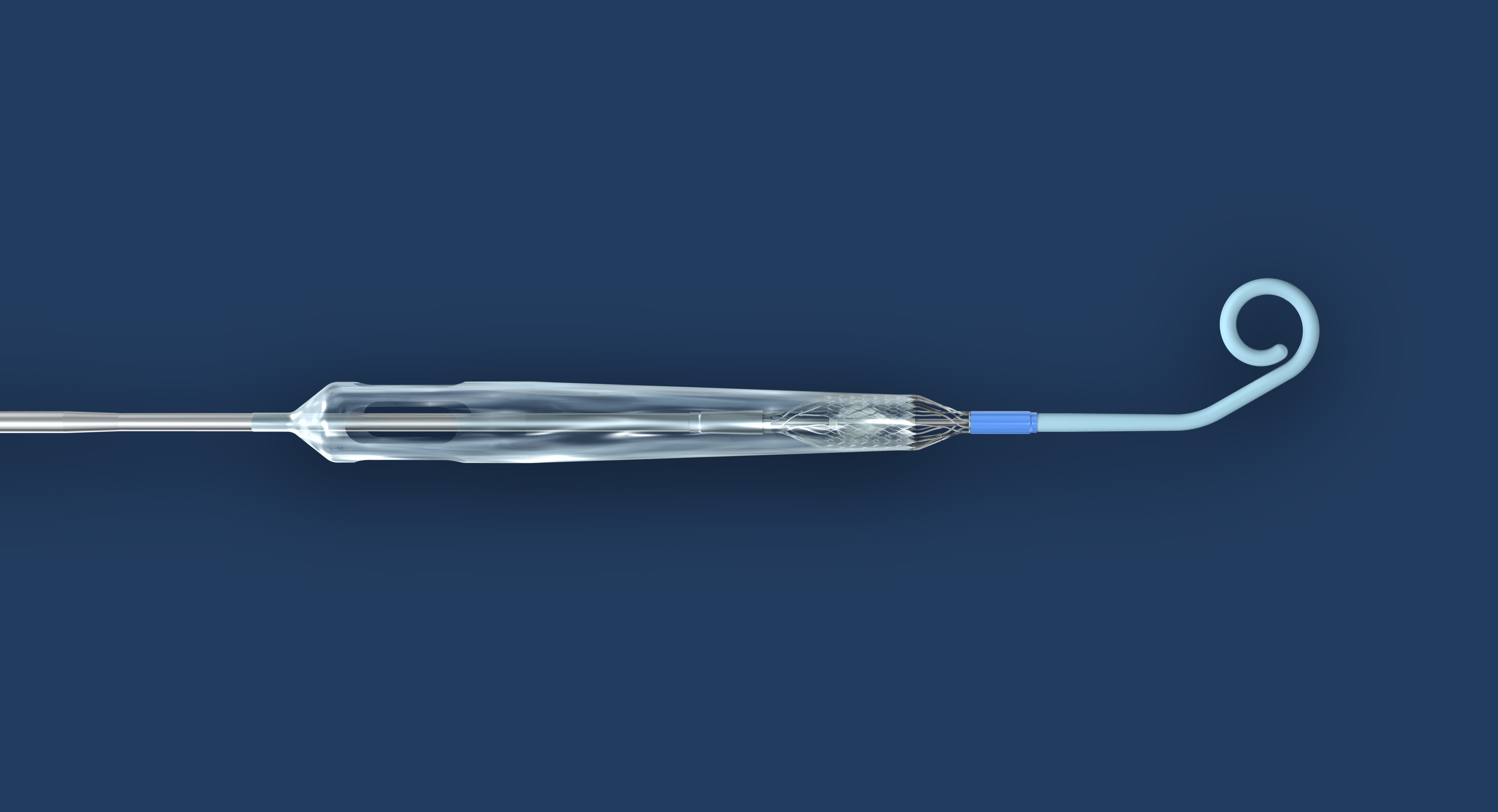
FDA Approves Impella ECP Pivotal Heart Pump and First Patients Enrolled in Pivotal Clinical Trial
December 21, 2022
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD


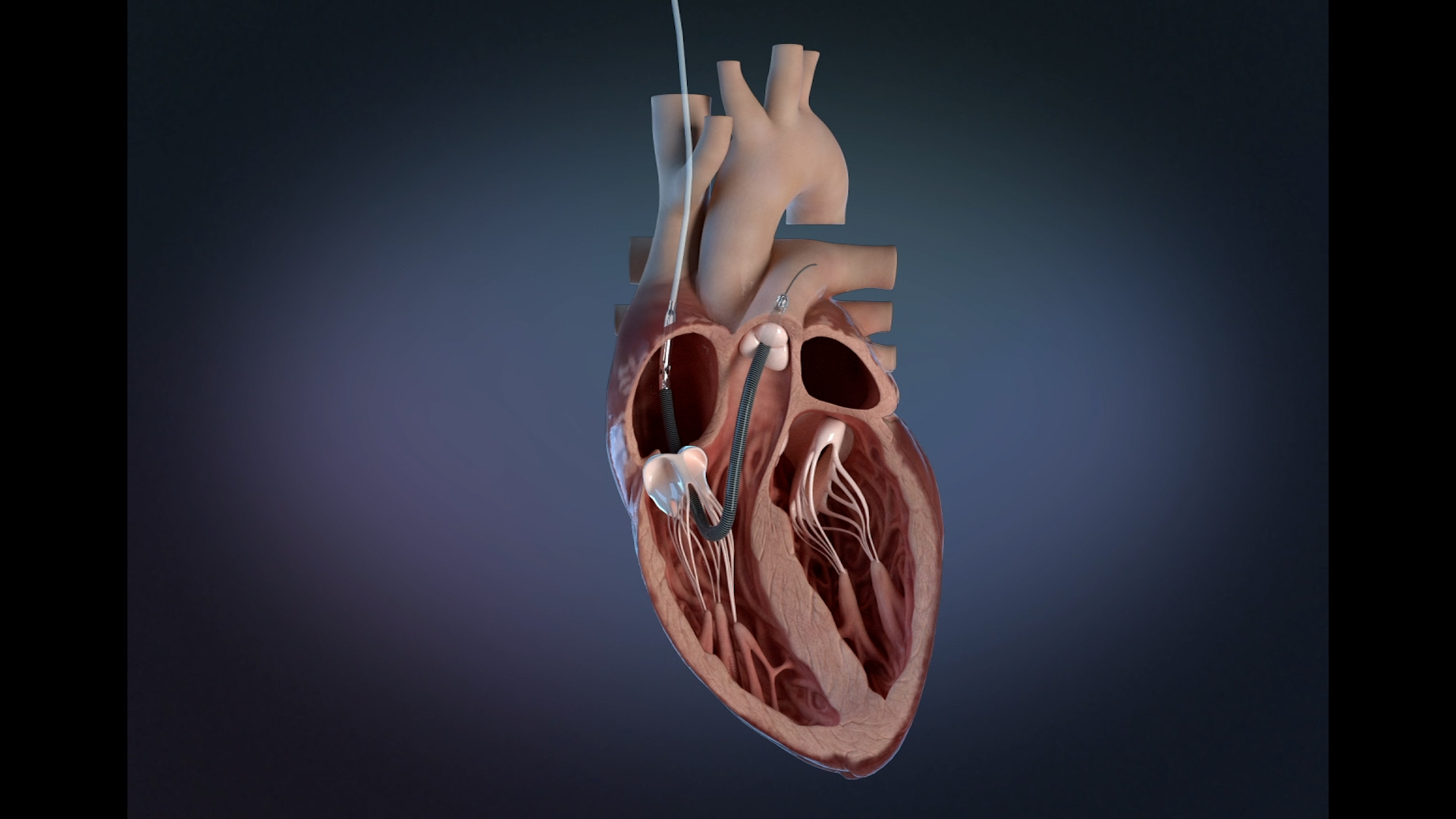
Impella RP Flex with SmartAssist Receives FDA Approval to Treat Right Heart Failure
October 31, 2022
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD

U.S. FDA Grants 510(k) Clearance for Impella Low Profile Sheath
October 17, 2022
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD

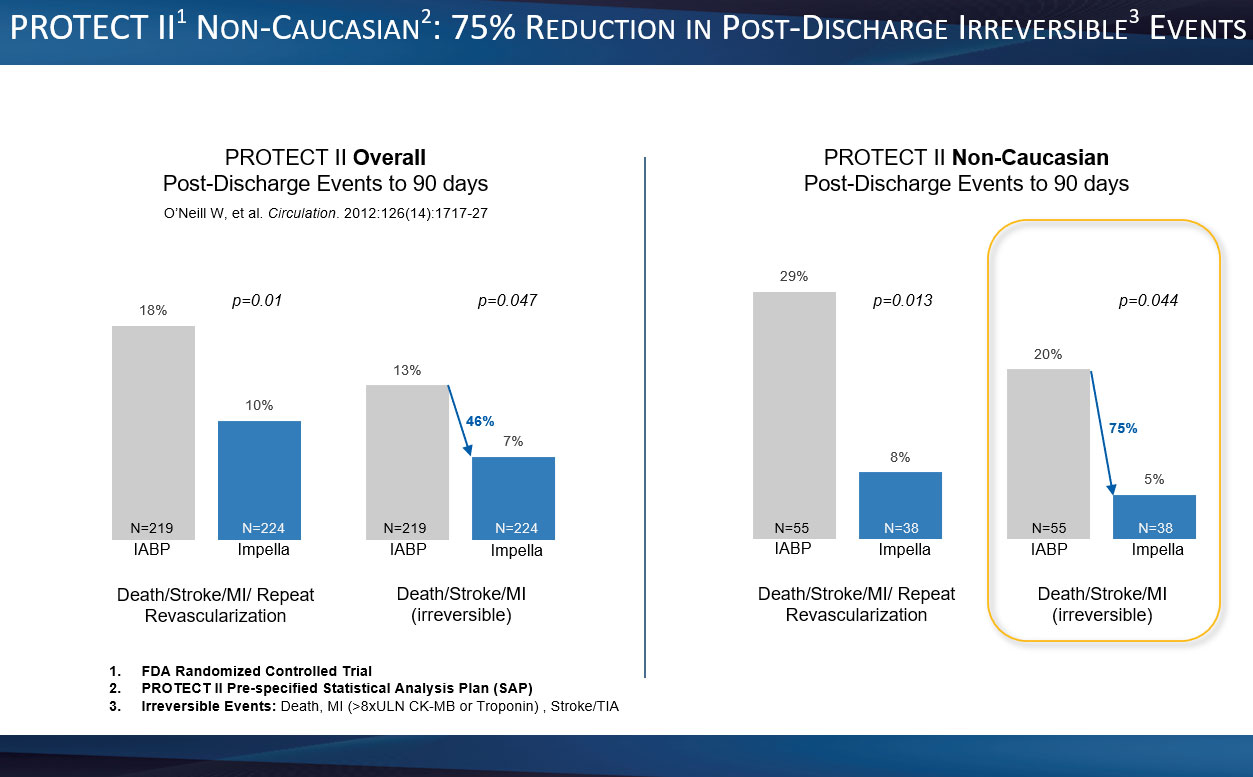
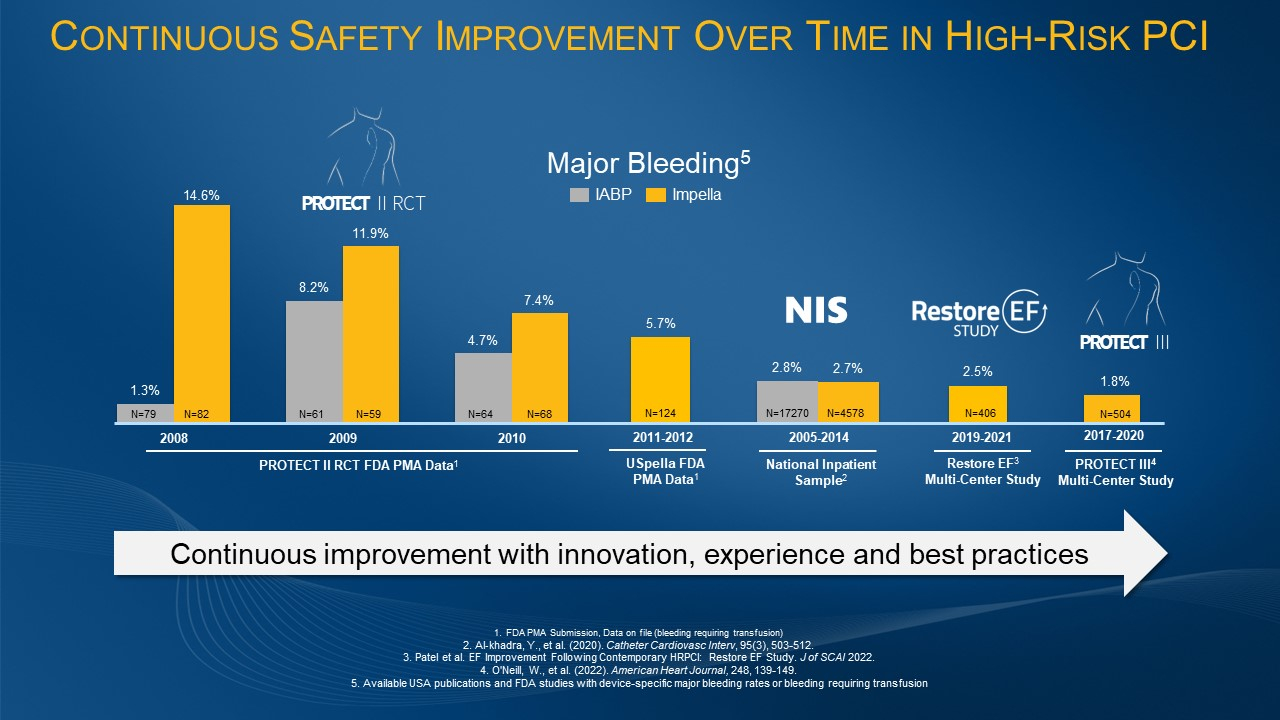
Yale University Study Demonstrates Significant Survival Benefit in High-Risk PCI with Impella Support
October 13, 2022
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD

From Abiomed, Inc.
Via Business Wire
Tickers
ABMD
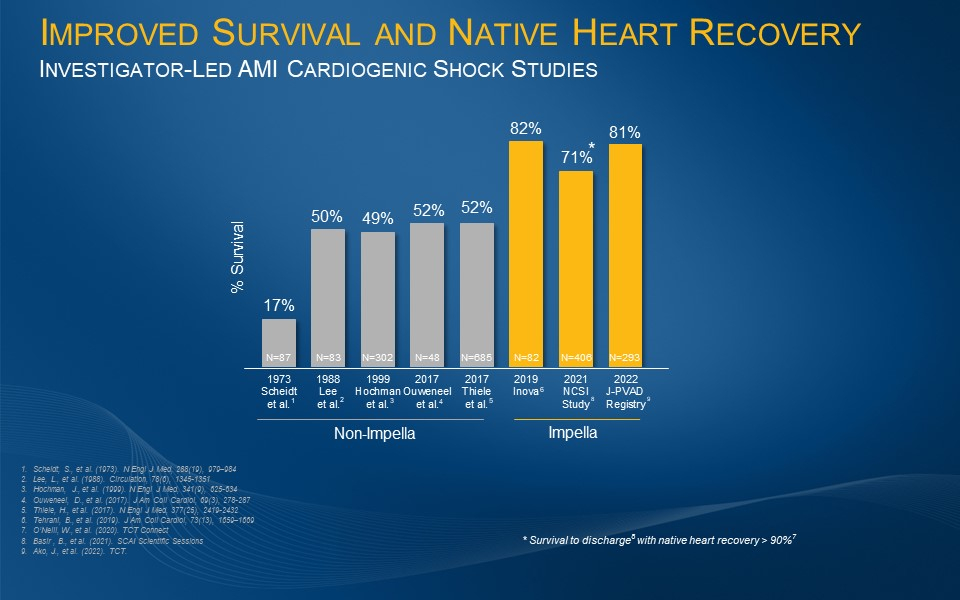
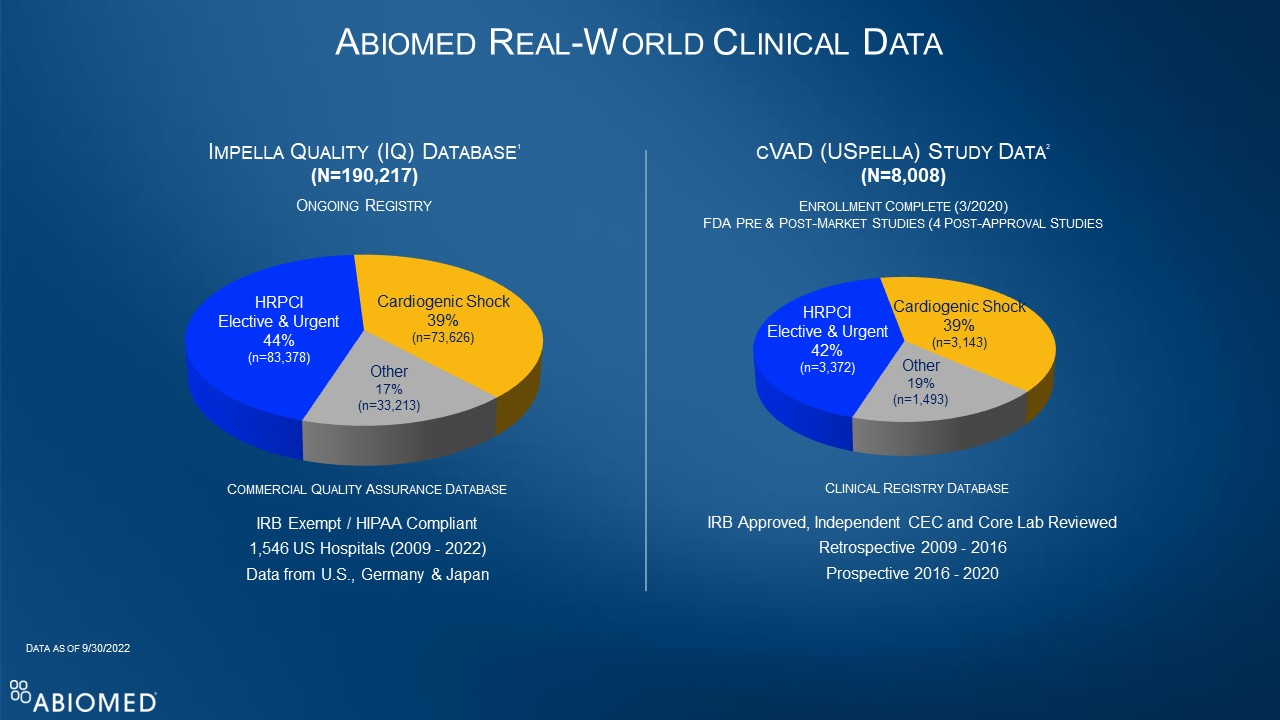
Large, Multi-Center, Multi-Society Study of Impella-supported Patients Finds 30-day AMI Cardiogenic Shock Survival of 81%
September 19, 2022
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD
Unloading with Impella for 30 Minutes Before PCI Associated with Reduced Infarct Size in STEMI Patients
September 17, 2022
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD

FDA Approves RECOVER IV Randomized Controlled Trial with Exception from Informed Consent (EFIC)
September 16, 2022
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD
TCT 2022: Impella Enables Complete Revascularization, Improved Quality of Life and Native Heart Recovery
September 13, 2022
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD


From Abiomed, Inc.
Via Business Wire
Tickers
ABMD

Abiomed to Host Investor Call on Heart Failure Opportunity With Impella 5.5 and Impella BTR Heart Pumps
June 14, 2022
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD

From Abiomed, Inc.
Via Business Wire
Tickers
ABMD

Abiomed Announces Fourth Quarter Record Revenue of $270 Million, up 12% Year Over Year
April 28, 2022
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD

From Abiomed, Inc.
Via Business Wire
Tickers
ABMD
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD

Abiomed to Host Investor Call on Impella ECP, the World’s Smallest Heart Pump at 9 French
March 21, 2022
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD

From Abiomed, Inc.
Via Business Wire
Tickers
ABMD

Abiomed Announces Record Revenue of $261 Million, up 13% Year Over Year
February 03, 2022
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD

From Abiomed, Inc.
Via Business Wire
Tickers
ABMD
Early Feasibility Study Demonstrates Successful Use of Abiomed’s preCARDIA Technology
January 12, 2022
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD

Abiomed to Present at the 40th Annual J.P. Morgan Healthcare Conference
December 29, 2021
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD

Abiomed to Present at Upcoming Investor Conferences
November 18, 2021
From Abiomed, Inc.
Via Business Wire
Tickers
ABMD
Stock Quote API & Stock News API supplied by www.cloudquote.io
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.
© 2025 FinancialContent. All rights reserved.
